
- Critical Care Services
- Critical Care Guidelines
- Renal, Metabolic and Endocrine
- Antimicrobial dosing in renal failure

INTRODUCTION
Renal Replacement Therapy (RRT) is widely used to support critically ill patients with renal failure1. This can clearly take a multitude of variations: acute, acute-on-chronic, or established chronic renal failure. Acute renal failure (ARF) is defined as a sudden, sustained decline in glomerular filtration rate, usually associated with uraemia and a fall in urine output2. Most patients with ARF do not require RRT3. The decision to commence RRT is made on an individual patient basis, with several factors contributing to the final decision to start, such as urine output, acid-base balance and uraemia.
RRT can take several forms: established intermittent haemodialysis (via a temporary or semi-permanent dialysis catheter or arterio-venous fistula); continuous arterio-venous haemodialysis (CAVH) or continuous veno-venous haemodialysis (CVVH); peritoneal dialysis (PD) and ultimately renal transplant. CVVH is frequently used to treat critically ill patients with ARF or chronic renal failure (CRF)1, is better tolerated by haemodynamically unstable patients and is as effective at removing solutes during a 24-48 hour period as a single session of conventional haemodialysis4.
The use of drugs in patients with renal failure can give rise to problems for a number of reasons1, 5. Complex pharmacokinetics dictates how a drug is eliminated in critically ill patients. Given the large number of variables involved generalized dosing schedules are difficult to recommend. Antimicrobials with a low protein-binding capacity in serum are removed by CRRT (continuous RRT) more readily. Antimicrobials binding to tissues have a large volume of distribution and will thereby reduce the amount removed during CRRT. Sepsis, which is a common problem in the critical care setting, can alter the drug’s volume of distribution, half-life and protein-binding capacity 1.
Other factors to consider are the mechanical factors involved with RRT in the critical care setting. Blood and dialysate flow rates together with changes in transmembrane pressure can alter clearance, as can dialysate concentration and membrane pore size1.
There are no recent comprehensive guidelines currently that have a strong evidence-base for antimicrobial dosing in patients receiving RRT in the critical care setting. Furthermore it can be argued that the dose used for a patient on intermittent haemodialysis will be different to the dose for a patient on CVVH. There is relatively little in the way of double-blind randomised-controlled trials to determine the “evidence-based” appropriate dose; what follows is a brief synopsis of the antimicrobials used at the James Cook University Hospital Intensive Care Unit with a dosing suggestion based on current literature and other guidelines used within other regions in the UK.
GLOMERULAR FILTRATION RATE (GFR)
The antimicrobial dose administered to the patient will depend primarily on the severity of renal failure. This can be established in various ways and the primary method is by the Cockroft-Gault equation. This equation is a commonly used surrogate for actual creatinine clearance, employing serum creatinine measurements and the patient’s mass and age. This gives an estimation of the Glomerular Filtration Rate (GFR). A considered normal value is a GFR of >120 ml/min.
There are limitations to the use of this, for example, patients with a reduced muscle mass may have a GFR of < 50 ml/min without a highly elevated creatinine, or indeed those with a rapidly changing creatinine level.
Renal function is increasingly being reported on “routine” blood chemistry results by way of an estimated GFR (eGFR). This is based on another method of calculating the GFR and normalising the result for body surface area of 1.73 m2. This is derived from the Modification of Diet in Renal Disease (MDRD) formula. This formula uses serum creatinine, age and sex, however, in contrast to the Cockroft-Gault equation, will also factor in the patient’s race and the body surface area.
An absolute GFR can be calculated by dividing the product of the eGFR and the patient’s body surface area by 1.73. For prescribing purposes the British National Formulary has divided renal function arbitrarily into three distinct groups based on the GFR(absolute), namely: mild (GFR(absolute) 20-50 ml/min), moderate (10-20 ml/min) and severe (<10 ml/min)6.
CRRT is equivalent to a GFR of 25-50 ml/min; clearly flow rates and high volume filtration (>3000 ml/hr) may increase the GFR. This will have an effect on antimicrobial clearance.
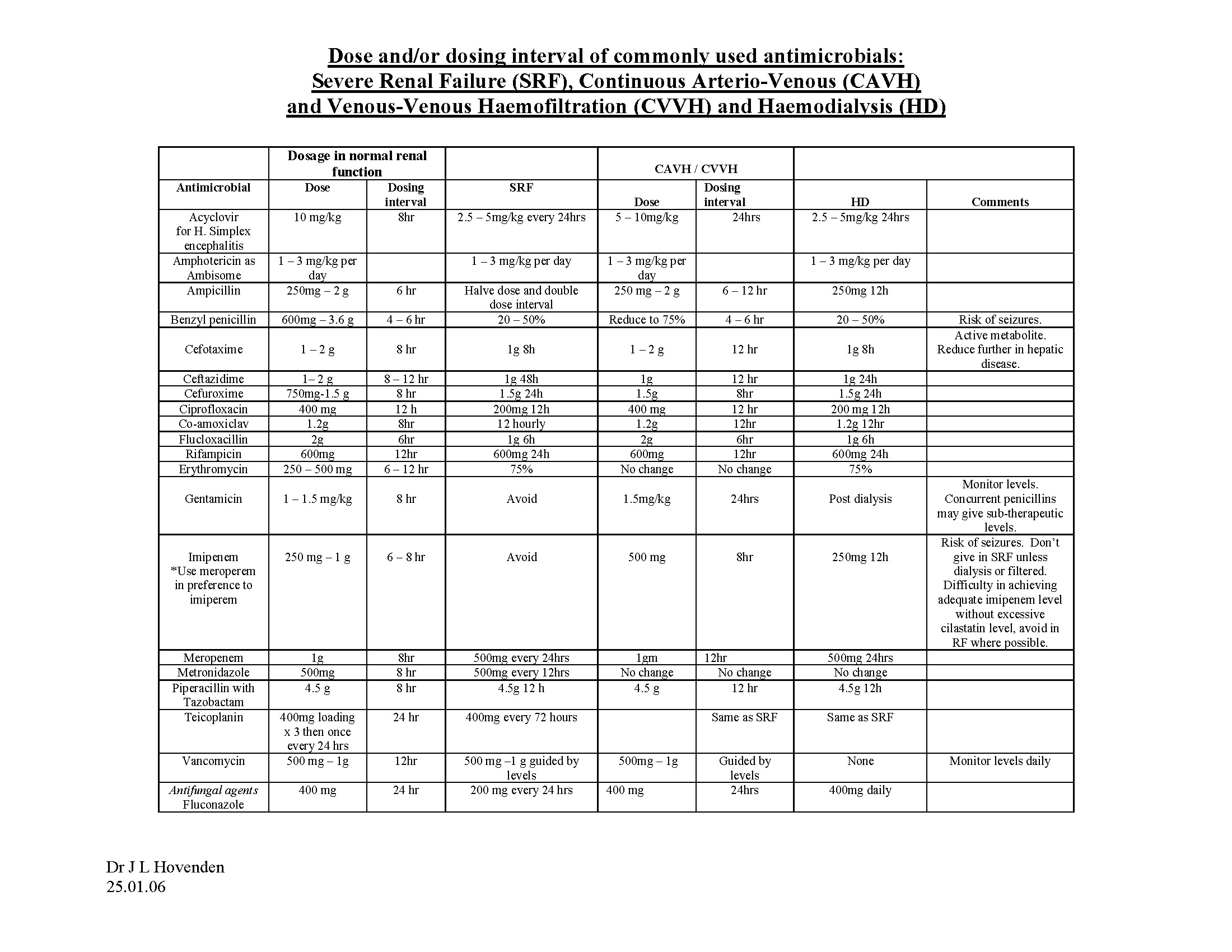
ANTIMICROBIALS USED AT THE JCUH
Acyclovir (IV)
- An antiviral agent used for treating herpes simplex and herpes zoster infections.
- Dose in normal or immunosuppressed patients:
Herpes simplex: 5mg/kg, 8 hourly.
Herpes simplex encephalitis: 10mg/kg, 8 hourly.
Primary and recurrent varicella zoster infections: 10mg/kg, 8 hourly.
- Dose in renal impairment (GFR, ml/min):
Mild: 5-10mg/kg, 12 hourly.
Moderate: 5-10mg/kg, 24 hourly.
Severe: 2.5-5mg/kg, 24 hourly.
- Acyclovir is not dialysed in CAPD.
- Patients on HD should have severe renal impairment dosing.
- Patients on CVVH should have moderate renal impairment dosing.
- Note that acyclovir can exacerbate renal impairment if infused too quickly; ideally infuse over 1-hour.
- This can be reconstituted in either 0.9% sodium chloride or 5% dextrose.
- Acyclovir is eliminated by renal excretion and should be given post dialysis.
Acyclovir (oral)
- Dose in normal renal function:
Herpes simplex: 200-400mg 5 times daily.
Prophylaxis: 200mg, 6 hourly.
Suppression: 200mg 6 hourly or 400mg 12 hourly.
Herpes zoster: 800mg 5 times daily for 7 days.
- Dose in renal impairment:
Mild: Dose as in normal renal function.
Moderate: Simplex dose: as in normal renal function.
Zoster dose: 800mg 8 hourly.
Severe: Simplex dose: 200mg, 12 hourly.
Zoster dose: 800mg, 12 hourly.
- As with IV administration, patients on HD should have severe renal impairment dosing and those on CVVH should have moderate renal impairment dosing. Oral acyclovir is not dialysed in CAPD.
- Doses should be given post dialysis.
Amikacin
- Aminoglycoside drug indicated in use for serious Gram-negative infections resistant to gentamicin.
- Dose in normal renal function: 15mg/24 hours, in two divided doses (maximum dose of 1.5g/day with a maximum total dose of 15g). Daily dose may be increased to 22.5mg/kg in three divided doses in severe infections.
- Serum levels must be measured for efficacy and toxicity.
- One-hour peak serum concentrations should not exceed 30mg/l, with pre-dose trough levels no lower than 10mg/l.
- Dose in renal impairment:
Mild: 5-6mg/kg, 12 hourly.
Moderate: 3-4mg/kg, 24 hourly.
Severe: 2mg/kg, 24-48 hourly.
- Amikacin is eliminated by renal excretion and therefore must be given post dialysis.
- Patients on CAPD should have dose as in severe renal impairment.
- Patients on HD should have a dose of 5mg/kg post dialysis.
- Patients on CVVH should have dose as in moderate renal impairment, with serum concentration monitoring.
- Note there is an increased risk of ototoxicity with concomitant use with loop diuretics.
Amoxycillin (IV)
- Penicillin used in oral infections and endocarditis; adjunct in listerial meningitis and H. pylori eradication.
- Dose in normal renal function: 250-500mg, 8 hourly; maximum dose: 6g/24 hours. Dose may be doubled in severe infections.
- Dose in renal impairment:
Mild: as normal renal function.
Moderate: as normal renal function.
Severe: as normal renal function 12 hourly.
- Patients undergoing CAPD or HD should have dose reduced as in severe renal failure.
- Patients undergoing CVVH should have dose as in normal renal function.
- Amoxycillin is eliminated by renal excretion and should be given post dialysis.
Amoxycillin (oral)
- Dose in normal renal function is as IV dose.
- Dose in renal impairment:
Mild: as IV dose.
Moderate: as IV dose.
Severe: 250mg, 8 hourly.
- As IV administration, oral amoxicillin ought to be given post dialysis.
Amphotericin
- A polyene antifungal agent.
- Not absorbed when given by mouth.
- Used in the treatment of systemic fungal infections and is active against most fungi and yeasts.
- IV administration commonly leads to side-effects; lipid formulations of amphotericin are significantly less toxic and are recommended when conventional formulation amphotericin is contra-indicated because of toxicity, especially nephrotoxicity.
- Dose in normal renal function:
Normal agent: 250µg-6mg/kg/24 hours (usually in range 500µg-1.5mg/kg/24 hours)
Liposomal amphotericin: 1-5mg/kg/24 hours.
- Dose in renal impairment:
Mild: 250µg-6mg/kg 24 hourly.
Moderate: 250µg-6mg/kg 24 hourly.
Severe: 250µg-6mg/kg 24-36 hourly.
- This drug is not dialysed, however those on CAPD and HD ought to have their dose adjusted as severe renal impairment.
- Those on CVVH ought to have their dose as moderate renal impairment.
- Liposomal amphotericin may be infused over 1 hour, however, standard amphotericin must be infused over 6 hours.
- The IV line must be flushed with 5% dextrose pre- and post-administration.
- Those on CVVH must have the drug given in the venous return of the dialysis circuit.
- A test dose must be given prior to a full course starting.
- Essential to monitor renal function, FBC, K, Mg and Ca levels.
Benzylpenicillin
- A penicillin used in throat infections, otitis media, endocarditis, meningococcal disease, pneumonia and cellulitis
- Dose in normal renal function: 600-14.4g/24 hours given in 2-6 divided doses.
- Dose in renal impairment:
Mild: dose as normal renal function.
Moderate: 75% normal renal function dose.
Severe: 20-50% normal renal function dose.
- Benzylpenicillin is dialysed, and must therefore be given post dialysis.
- Those on CAPD or HD must have their dose reduced as severe renal impairment.
- Those on CVVH must have their dose reduced as moderate renal impairment.
- The maximum dose in severe renal impairment is 2.4-3.6g/24 hours.
- There is an increased risk of neurotoxicity and seizures in renal impairment.
Cefotaxime
- Third generation cephalosporin.
- Dose in normal renal function:
Mild infection: 1g 12 hourly
Moderate infection: 1g 8 hourly.
Severe infection: 2g 8 hourly
Life-threatening infection: up to 12g in 24 hours in 3-4 divided doses.
- Dose in renal impairment:
Mild: as in normal renal function.
Moderate: as in normal renal function.
Severe: 0.5-1g every 8-12 hours.
- Cefotaxime ought to be given post dialysis.
- Those undergoing CAPD and HD ought to have their dose adjusted as in severe renal impairment.
- Those undergoing CVVH ought to have 1g 12 hourly.
- Reduce the dose if there is concurrent hepatic failure.
Ceftazidime
- Third generation cephalosporin and good anti-pseudomonal agent.
- Dose in normal renal function: 1-2g 8-12 hourly.
- Dose in renal impairment:
Mild: 1g 12-24 hourly
Moderate: 500mg-1g 24 hourly
Severe: 500mg-1g 48 hourly
- Patients undergoing CAPD should have the dose adjusted to 500mg-1g 14 hourly
- Patients undergoing HD should receive 500mg-1g 24-48 hourly.
- CVVH patients may have 500mg-1g 12 hourly.
- Should be administered post dialysis.
Ceftriaxone
- Third generation cephalosporin with a longer half-life and can be given once daily.
- Indicated in serious infections such as septicaemia and meningitis.
- Dose in normal renal function: 1g 24 hourly (with dose increase to 2-4g if very severe).
- Dose in renal impairment:
Mild/Moderate/Severe: dose as in normal renal function.
- The clearance of ceftriaxone is not clearly known, though thought to be equivalent to that of someone with normal renal function. The dose for patients undergoing CAPD/HD and CVVH should remain as that in normal renal function.
- In severe renal impairment, the maximum daily dose should be capped at 2g in 24 hours.
Cefuroxime
- Second generation cephalosporin, with greater activity against Haemophilus influenzae.
- Used in surgical prophylaxis
- Dose in normal renal function: 750mg-1.5g 6-8 hourly.
- Dose in renal impairment:
Mild: 750mg-1.5g 8 hourly.
Moderate: 750mg-1.5g 8-12 hourly
Severe: 750mg-1.5g 24 hourly
- The dialysability of cefuroxime is unknown, and the dose for those on CAPD and HD should be adjusted as per severe renal impairment.
- Those on CVVH should have a dose adjustment as moderate renal impairment.
- Note that cefuroxime and metronidazole can be mixed.
- Cefuroxime should be given post dialysis.
Ciprofloxacin (IV and oral)
- A quinolone drug active against both Gram-positive and Gram-negative bacteria.
- Dose in normal renal function:
Oral: 250-750mg 12 hourly
IV: 100-400mg 12 hourly
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate: 50% normal renal function dose
Severe: 50% normal renal function dose.
- Patients receiving CAPD should have a dose adjustment:
Oral: 250mg 8-12 hourly
IV: 100mg 12 hourly
- Patients on HD should have a dose adjustment:
Oral: 250-500mg 12 hourly
IV: 100mg 12 hourly
- Patients on CVVH should have dose adjustment:
Oral: 500-750mg 12 hourly
IV: 200mg 12 hourly
- The bioavailability of both oral and IV preparations are approximately equivalent.
- Ciprofloxacin is dialysed, and should be given post dialysis.
Clarithromycin (IV and oral)
- Macrolide antimicrobial, an erythromycin derivative.
- Indicated in mild-moderate soft tissue infections and respiratory-tract infections. Also used in H. pylori eradication.
- Dose in normal renal function:
Oral: 250-500mg 12 hourly
IV: 500mg 12 hourly
- Dose in renal impairment:
Mild: as normal renal function
Moderate: Oral: 250-500mg 12-24 hourly
IV: 250-500mg 12 hourly
Severe: Oral: 250mg 12-24 hourly
IV: 250mg 12 hourly
- Those on CAPD and HD should have a dose adjustment as for severe renal impairment.
- Those on CVVH should have a dose adjustment as for moderate renal impairment.
- The dialysability of clarithromycin is not clearly known.
Clindamycin (IV and oral)
- Active against Gram-positive cocci including streptococci and penicillin-resistant staphylococci.
- Also active against many anaerobes, especially Bacteroides fragilis.
- Excreted in bile and urine.
- Can also be used in MRSA infections in bronchiectasis and bone and joint infections.
- Dose in normal renal function:
Oral: 150-450mg 6 hourly
IV: 0.6-4.5g in 24 hours in 2-4 divided doses.
- Dose in renal impairment:
Mild/Moderate/Severe: dose as in normal renal function.
- Patients receiving RRT by any of the common methods should receive a dose equivalent to that of someone with normal renal function.
Co-Amoxiclav (IV and oral)
- A mixture of the penicillin amoxicillin and clavulanic acid.
- Dose in normal renal function:
Oral: 375mg 8 hourly, maximum 625mg 8 hourly
IV 1.2g 8 hourly (may increase to 6 hourly if severe)
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate:
Oral: 375mg or 625mg 8-12 hourly
IV: 1.2g stat, followed by 600mg 12 hourly
Severe:
Oral: 375mg 8-12 hourly
IV: 1.2g stat followed by 600mg 24 hourly
- In patients receiving CAPD or HD the dose should be adjusted as for severe renal impairment.
- In those receiving CVVH, adjust the dose as for moderate renal impairment.
- Dose should be given post dialysis.
Co-Trimoxazole
- A combination therapy of a sulphonamide and trimethoprim, used in the treatment of Pneumocystis jiroveci (prev P. carinii) pneumonia.
- Contra-indicated in patients with porphyria.
- Dose in normal renal function:
Oral: 60mg/kg 12 hourly
IV: 60mg/kg 12 hourly
- Dose in renal impairment:
Mild: dose is as in normal renal function
Moderate: 60mg/kg 12 hourly for 3 days, then 30mg/kg 12 hourly
Severe: 60mg/kg 24 hourly or 30mg/kg 12 hourly (only if HD facilities are available)
- If patients are receiving CAPD or HD, dose adjustment as for severe renal impairment.
- If undergoing CVVH, dose adjustment as for moderate renal impairment.
- Administer the drug post dialysis.
Erythromycin (IV and oral)
- A macrolide and parent of clarithromycin.
- Antibacterial spectrum is similar to that of penicillin.
- Dose in normal renal function:
Oral: 250-500mg 6 hourly or 0.5-1g 12 hourly
IV: 25mg/kg/24 hours (mild to moderate) or 50mg/kg/24 hours (severe). Maximum dose 4g/24 hours
- Dose in renal impairment:
Mild: dose is as in normal renal function
Moderate: dose is as in normal renal function
Severe: 50-75% of normal renal function dose with a maximum of 1.5g/day.
- Those on CAPD and HD should have a dose as per severe renal impairment.
- CVVH patients should have a normal renal function dose.
- Erythromycin has an unknown dialysability in CAPD and CVVH. It is not dialysed in HD.
Flucloxacillin (IV and oral)
- A penicillin used in cellulitis, osteomyelitis and in staphylococcal endocarditis.
- Dose in normal renal function:
Oral: 250-500mg 6 hourly
IV: 250mg-2g 6 hourly
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate: dose as in normal renal function
Severe: dose as in normal renal function with a total daily dose of 4g
- CAPD/HD patients should have dose adjustment as per severe renal impairment.
- CVVH patients should receive the normal renal function dose.
- Flucloxacillin is not dialyzable.
Fluconazole
- A triazole antifungal agent, well absorbed after oral administration. Also has good CSF penetrance in treating fungal meningitis.
- Dose in normal renal function: 50-400mg 24 hourly.
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate: dose as in normal renal function
Severe: 50% normal renal function dose
- CAPD/HD patients should have dose reduction as per severe renal function.
- CVVH patients should receive normal renal function dose.
- Due to the very high bioavailability, the oral dose is equivalent to the IV dose.
- Fluconazole is dialysed in all modes of RRT, and should be given post dialysis.
Fusidic Acid
- Indicated in infections caused by penicillin-resistant staphylococci, and well concentrated in the bone.
- Dose in normal renal function:
Oral: 480mg 8 hourly
IV: 480mg 8 hourly
- Dose in renal impairment:
Mild/Moderate/Severe: dose as normal renal function
- CAPD/HD/CVVH patients should receive the normal renal function dose.
- Fusidic acid is not dialysed.
Gentamicin
- An aminoglycoside drug active against some Gram-positive and many Gram-negative organisms.
- The dosing may be delivered as once-daily or multiple dose regimens.
- Dose in normal renal function can be based on the Hartford nomogram (single daily dosing), but is generally accepted as 3-7mg/kg, 8 hourly.
- Dose in renal impairment (for single daily dosing):
GFR>80: 5.1mg/kg 24 hourly
GFR 60-80: 4.0mg/kg 24 hourly
GFR 40-60: 3.5mg/kg 24 hourly
GFR 30-40: 2.5mg/kg 24 hourly
GFR 20-30: 4.0mg/kg 48 hourly
GFR 10-20: 3.0mg/kg 48 hourly
GFR<10: 2.0mg/kg 48 hourly
- The adjustment for renal impairment and dialysis is up to 2mg/kg, post-dialysis.
- Note that concurrent penicillin therapy may result in sub therapeutic blood levels.
- When using gentamicin, it is strongly recommended to seek Consultant Microbiological advice.
- Dose should be given post dialysis.
Meropenem
- Beta-lactam antibiotic used in CNS infections, active against aerobic and anaerobic Gram-positive and Gram-negative infections.
- Dose in normal renal function: 0.5-1g 8 hourly
- Dose in renal impairment:
Mild: 0.5-1g 12 hourly
Moderate: 500mg 8 hourly
Severe: 0.5-1g 24 hourly
- HD patient should have dose adjustment as per severe renal impairment.
- CVVH patients should have dose adjustment as per mild renal impairment.
- Meropenem should be given post dialysis.
Metronidazole
- Antimicrobial active against anaerobic bacteria and protozoa.
- Dose in normal renal function:
Oral: 400mg 8-12 hourly
IV: 500mg 8 hourly
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate: dose as in normal renal function
Severe: normal dose 12 hourly
- CAPD patients should have dose adjustment as per severe renal impairment.
- HD and CVVH patients should receive a normal renal function dose.
- Metronidazole is not dialysed via CAPD, is dialysed via HD and has an unclear dialysability in CVVH.
- Give post dialysis.
Piperacillin and Tazobactam (Tazocin®)
- Ureidopenicillin (piperacillin) and beta-lactamase inhibitor combination.
- Dose in normal renal function: 4.5g 8 hourly
- Dose in renal impairment:
Mild: 4.5g 8 hourly
Moderate: 4.5g 12 hourly
Severe: 4.5g 12 hourly
- CAPD and HD patients should have dose adjustment as per severe renal impairment.
- CVVH patients should have dose adjustment as per moderate renal impairment.
- Piperacillin and tazobactam are dialysed and should be given post dialysis.
Rifampicin (IV and oral)
- A rifamycin drug used in tuberculosis, brucellosis, legionnaire’s disease and serious staphylococcal infections. Also used in the prophylaxis of meningococcal meningitis.
- Dose in normal renal fuction: (IV and oral) 450-1200mg 24 hourly
- Dose in renal impairment:
Mild: dose as in normal renal function
Moderate: dose as in normal renal function
Severe: 50-100% of normal renal function dose.
- CAPD and HD patients should have a dose adjustment as per severe renal impairment.
- CVVH patients should receive a normal renal function dose.
Teicoplanin
- A glycopeptide antimicrobial used in potentially serious Gram-positive infections.
- Dose in normal renal function: 3-6mg/kg/24 hours (up to 12mg/kg/24 hours). Generally accepted to have a loading regimen of 400mg 12 hourly for three doses, then maintenance of 400mg daily.
- Dose in renal impairment:
Mild: 50% normal daily dose or normal daily dose 48 hourly
Moderate: 30% normal daily dose or normal daily dose 72 hourly
Severe: as for moderate renal impairment.
- CAPD and HD patients should have dose adjustment as per severe renal impairment.
- CVVH patients should have dose adjustment as per moderate renal impairment.
- For patients with renal impairment, a dose reduction is not necessary until day 4 of therapy.
Vancomycin
- Another glycopeptide antimicrobial used in Gram-positive infections.
- Important to monitor drug levels, in particular, trough or pre-dose levels to guide therapy.
- Dose in normal renal function: 1-1.25g 8-12 hourly
- Dose in renal impairment:
GFR>60: 1g 12 hourly
GFR 40-60: 1g 24 hourly
GFR 30-40: 750mg 24 hourly
GFR 20-30: 1g 48 hourly
GFR<20: 1g 72 hourly
- HD patients should have dose adjustment as per GFR<20 ml/min.
- CVVH patients should have dose adjustment as per GFR 40-60 ml/min.
- Recommended plasma concentration is 5-20mg/l.
- As with gentamicin dosing it is important to seek Consultant Microbiological advice.
REFERENCES
1. Trotman RL, Williamson JC, Shoemaker DM, Salzer WL. Antibiotic dosing in critically ill adult patients receiving continuous renal replacement therapy. Clin Infect Dis 2005;41:1159-66
2. Nissensen AR. Acute renal failure: definition and pathogenesis. Kidney Intl 1998;53:suppl 66 s7-10
3. Metcalfe W et al. Acute renal failure requiring renal replacement therapy: incidence and outcome. Q J Med 2002;95:579-83
4.Joy M, Matzke G, Armstrong D, Marx M, Zarowitz B. A primer on continuous renal replacement therapy for critically ill patients. Ann Pharmacother 1998;32:362-75
5. Bunn R, Ashley C (eds). The renal drug handbook. Radcliffe medical press. ISBN 1 85775 115 9
6. Martin J (Acting exec Ed). British national formulary, Volume 54 (September 2007)
7. Northern Deanery Antibiotic guidelines.